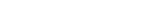Pre-Filled Syringe
Plastic Prefillable
ClearJect® Syringe
Cyclo Olefine Polymer (COP)

파트 명칭 및 재질
플라스틱 주사기
Clearject® COP
- 일본 TAISEI KAKO사의 플라스틱 프리필드 주사기
- 모든 공정 자동화 시스템 (Clean room 진행)
- Sterilization : 감마멸균 (Barrel, Tip cap, Piston)
- Barrel : Cyclo Olefine Polymer 소재 (감마멸균)
- Tip Cap / Piston : Butyl Rubber 소재 (감마멸균)
- ISO13485 인증 생산시설
- Damage / Cracking에 우수
- Siliconization : 12,500cSt. 의료등급 실리콘 사용
- Inspection : 100% 카메라 검사 수행
- Packing : Ready-To-Use (RTU) 포장
Cyclo Olefine Polymer (COP)
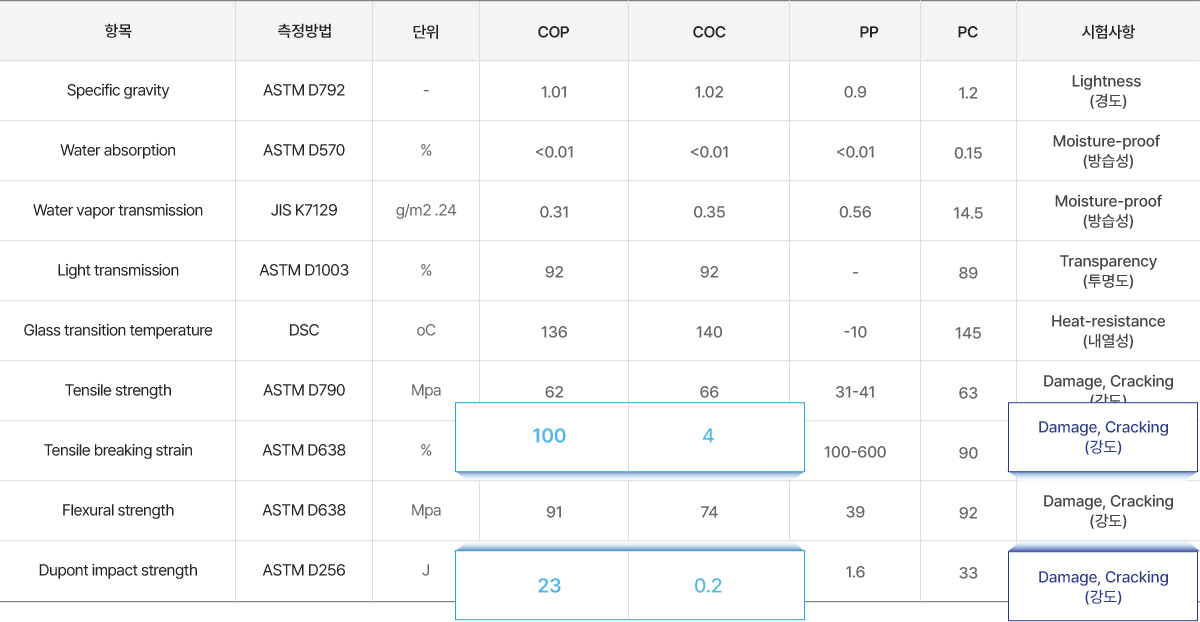
플라스틱 주사기 재질 특성 비교
감마 멸균 완료 플라스틱 주사기
Barrel(COP)
Cap/Piston(Butyl Rubber)

감마멸균 완료 후
Packing
- 다양한 멸균 호환 가능
-
AutoclaveEthylene Oxide GasElectron Beam
감마멸균 진행에 따른 Barrel (COP)의 색 변화

Non-irradiated barrel
25kGy
irradiation


One week has passed


Four weeks have passed
Biological safety test
생물학적 안정성 시험 통과 완료
Tested object
- ISO10993 Biological evaluation for Medical devices
- External communicating device, blood path, indirect ← 24h
Testing method
- COP-barrels, Butyl rubber pistons, PTDE-laminated pistons, SUS304-canulas
| Evaluation items | Judgement |
|---|---|
| Acute toxicity test with rats | Accepted |
| Skin sensitization with guinea pigs | Accepted |
| Cell toxicitytest with rabbits | Accepted |
| In vitro hemolysis toxicity test with rabbits blood | Accepted |
| Intradermal skin test with rabbits | Accepted |
| Pyrogen test with rabbits | Accepted |
Exractables Assessment
용출물 평가 시험 통과 완료
- USP1663
-
- -Assessment of Extractables Associated with Pharmaceutical
- -Packaging / Delivery Systems
Tested object
COP barrel, Butyl rubber piston, Teflon-film coating piston
Analysis
Extraction medium : aqueous, organic solvent etc.
Detected compounds
- γ-Ray irradiation sterilized COP barrel : Total over 34
- Autoclave-, γ-Ray irradiation- and autoclave-steriliazed butyl - rubber piston : Total over 47
- Twice autoclave-steriliazed Teflon-film coating piston : Total over 65
Toxicity assessment
Judgement as non-toxic material compared with toxicity data

ISO13485 인증 생산시설
ISO 인증 및 FDA 등록 완료
-
License Number: 27B2X00203 (1" Sep., 2015)
Marketing Authorization Holders for Second-Class Medical Device -
License Number: 27BZ200369 (21 May, 2015)
Medical Device Manufacturing License -
License Number: 27AZ200113 (21 May, 2015)
License for manufacturer of drugs (Sterile products) by MHLW
-
ISO13485:2016
Certification Number: JP19/040500 -
ISO 14001:2015 (SGS 14001:2015)
Certification Number: JP18/071554 -
DMF(Drug Master File) Registration for US-FDA
Manufacturing of Plastic Syringes: DMF21350
Manufacturing of Plastic Syringes with Needle: DMF29071
Ready-To-Use 포장


TASPACK®
ISO 8등급 환경에서 성형 되고, ISO 7등급 환경 자동화 시스템에서 포장되어, Barrel과 Tip-cap이 결합된 RTU (Ready-To-Use) 상태로 포장됩니다.